GalleryGallery
[CHOSUNILBO] Starting Localization of 'Insulin'...
-
- 2021-08-17
◈ Recruitment ‘Insulin Specialist’ Sahib of India
◈ Securing Price Competitiveness By Lowering Manufacturing costs By Producing High-Quality And High-Purity Products
◈ Plans To Sell At Domestic And Overseas From 2024
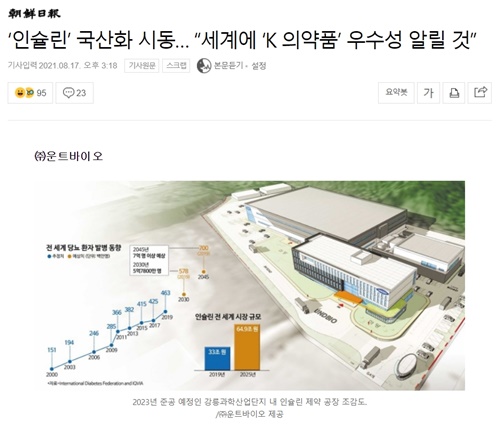
◈ Target Sales of KRW 1 Trillion and Operating Profit of KRW 500 Billion
▲ 2021. 08. 17. CHOSUNILBO Online News

▲ 2021. 08. 17. CHOSUNILBO Newspaper Article, KOSPI·KOSDAQ & Rising p.1
▷ Securing Competitiveness Through Research and Development on Insulin Commercialization and Production of High-Yield, High-Purity Products
Due to the expansion of cloned insulin market, insulin
commercialization R&D of UNDBIO Co., Ltd is attracting attention. UNDBIO
Co., Ltd is planning to produce insulin and such by securing original
technology combined with foreign technology according to global standards. Currently,
they are going through the initial research stage and even process of
commercialization. The company plans to complete raw material sample after
March of 2022 to compare and test the quality with advanced foreign products.
The challenges of insulin research and development include ▲establishing insulin producing gene recombinant lines (a set of cells that can continue to be cultured outside the body), ▲ developing optimal culture conditions, and ▲ securing high-purity raw material manufacturing technology that meets international quality standards. For this, the company has recruited Dr. Maharaj Kishen Sahib as a Chief of Research and Development, who had participated in insulin R&D for 15 years at a famous Indian pharmaceutical company. If the existing insulin technology of pharmaceutical companies are in the early stages of at least 15 years ago, Dr. Sahib's way of producing insulin is a combination of the latest technology developed between 2019 and 2020. By producing high-yield · hihj-purity products, the company can lower the manufacturing costs and secure global price competitiveness.
▷ Securing manpower and completing factory to expand the market
UNDBIO Co., Ltd recruited Vice Chairman Kwon Hyukjin, who has abundant experience in pharmaceutical RA and R&D planning, who also was the vice president of CELLTRION Pharmaceuticals R&D development and Dr. Soma Sekhar Penumajji, who is overseas license specialist.
After the completion of the plant in 2023, UNDBIO Co., Ltd plans to sell insulin produced by themselves in domestic and foreign markets from 2024 with Good Manufacturing Practice certification from Europe and the United States and product approval by country. It is expected to generate 1 trillion won in sales and 500 billion won in operating profit in the future.
▷ A large investment for the top priority, “health of the mankind.”
UNDBIO Co.,
Ltd places the highest value on achieving humanity’s desire to be free from
disease. To achieve these corporate objectives, the company will establish an
insulin biosimilar plant that meets U.S. and Europe GMP standards in the
Gangneung Science and Industry Complex in 2023. Stage by stage, the company is
planning to expand from general freeze-dry injections and contrast media to
anti-cancer drug processes. To this end, the company is also planning various strategies
such as cooperation with foreign pharmaceutical supply chain to attract investment.
Investment in insulin and biosimilar project is worth about 150 billion won including construction and product development costs. Among them, the cost of insulin raw materials and finished production facilities is about 80 billion won and about 37 billion won is planned to be invested in clinical trials for global insulin registration. The biosimilar portfolio expansion will be pursued in stages in consideration of future market prospects and investment adequacy. In the future, the company is considering listing not only on the domestic KOSDAQ, but also on the NASDAQ with a group specializing in distributing insulin products in the U.S.
Source: CHOSUNILBO
More on the article web link: Starting Localization of ‘Insulin’… “To Promote The Excellence of ‘K-Medicines’ To The World”